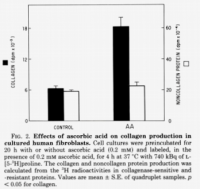
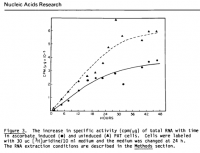
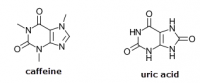
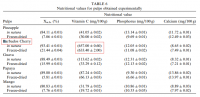
@Travis, I read somewhere that primates compensate for its inability to produce vitamin C by producing uric acid. And in one of the threads of haidut, it was mentioned that people with high amounts of uric acid, to the point of having gout, have little risk of getting cancer. I was talking to a friend who has breast cancer, and I mentioned this tidbit about uric acid and cancer, and she confirmed that she indeed has very low uric acid.Pauling argues that humans need much more. If you were to take the amount synthesized in the body by animals such as cats and dogs and extrapolate this onto humans who cannot make their' own, it is much more than what is commonly eaten except for some raw vegans perhaps.
Stay away from refined foods like rice and wheat and heat-treated foods. This way, you should get enough, or near enough. Ascorbate is heat-labile, so even pasteurized pineapple juice will have near zero ascorbate unless it is added afterwards.
I think he is correct, but a bane to the medical and food industries exactly for this reason.
Nobody can refute him. He was one of the most brilliant chemists in history. I adhere to his theory on cardiovascular disease 100%. I even did some fact-checking on lysine and lipoprotein A and it checks out. Theses two molecules do have a very high affinity for each other and bond 100% to each other when Lp(a) is passed through a chromatography column packed with lysine-doped media.
For those who haven't read that link, his theory on cardiovascular disease involves lipoprotein A bonding to lysine residues of collagen that hasn't been crosslinked. Citamin C is necessary to crosslink the collagen, this is beyond dispute. Pauling argues that lipoprotein A is actually a defense mechanism which plugs leaky vessels in the absence of vitamin c.
This helps explain why some carnivores cannot get atherosclerosis; they all biosynthesise their own vitamin C endogenously.
What came to my mind is what she could do to increase uric acid. I thought she could take a lot of fructose, but I didn't think it would just by itself produce enough uric acid in so short a time. I thought maybe she needed some blood vessel constriction mechanism to create hypoxic conditions so that uric acid production can be stimulated. But that would be too traumatic and dangerous. Now I'm thinking that megadosing with Vitamin C might be more appropriate, given that maybe the lack of uric acid can be compensated by megadosing with Vitamin C.
What are your thoughts?
Last edited: